How many unpaired electrons in cu+4?
1 Answer
May 3, 2018
There are three unpaired electrons.
Explanation:
I don't think that copper exists naturally in the +4 oxidation state.
However, it is theoretically possible to generate a
The electron configuration of copper is
For a +4 ion, we remove the
So, the electron configuration of the ion is
The electron configuration diagram becomes
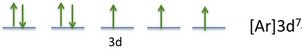
(Adapted from http://galleryhip.com/electron-configuration-diagram.html)
Thus, there are three unpaired electrons.

