How to calculate the density of this crystal?
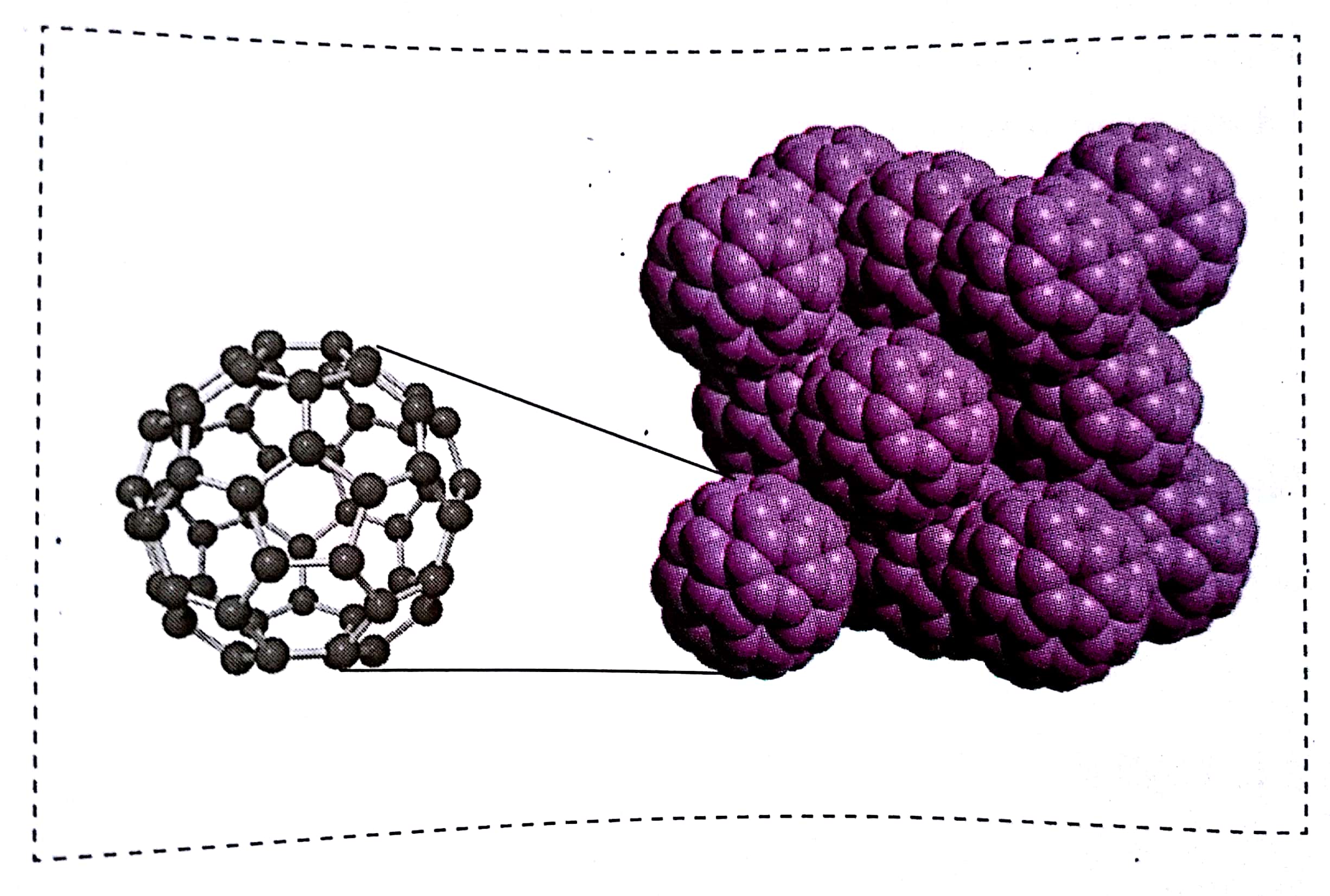
Fuleren is one of the most famous allotropic modification of carbon. The most famous is the fuleren is #C_60# . With an outer radius of 50.9 pm, sixty carbon atoms are connected to 20 hexagons and 12 spaced pentagons. This fuleren crystallizes in face-centered cubic system. How much is its density?

Fuleren is one of the most famous allotropic modification of carbon. The most famous is the fuleren is
1 Answer
The calculated density is
Explanation:
Calculate the molecules in a unit cell
The image in your question shows an fcc unit cell of fullerene.
There is ⅛ molecule at each of the 8 corners and ½ molecule at each of the six faces.
Thus, the number of molecules in a unit cell is
Calculate the mass of a unit cell
Calculate the edge length of a unit cell

Using Pythagoras' Theorem, we find
I believe your radius of
A more reasonable value is 509 pm.
Then
Calculate the volume of a unit cell
Calculate the density of the unit cell

