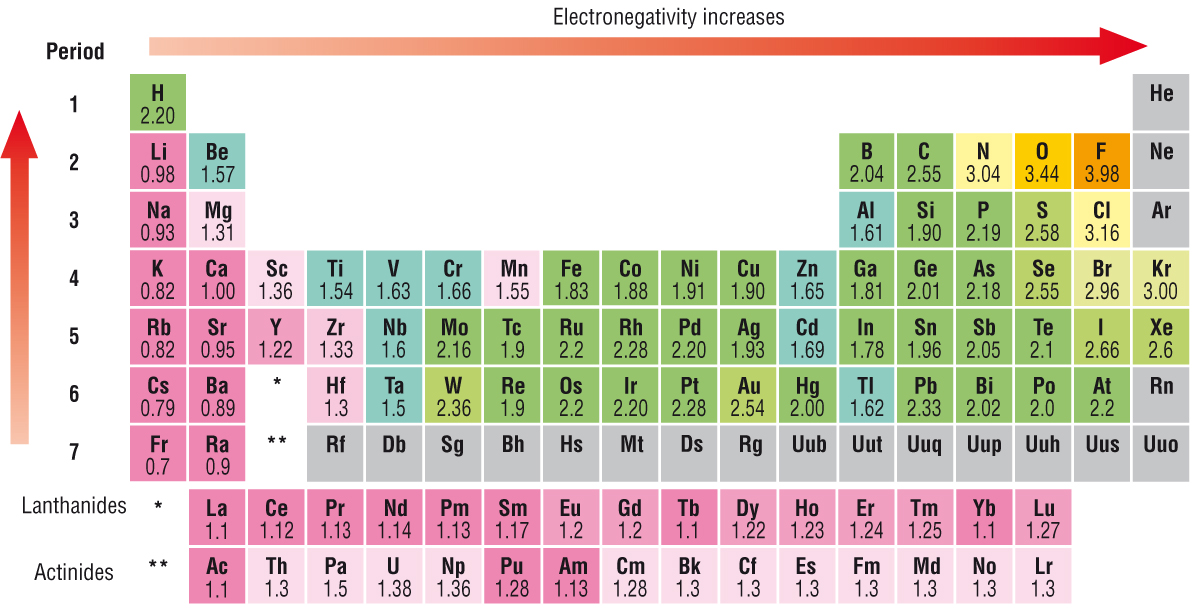
How would you arrange the following atoms in order of decreasing electronegativity using only the Periodic Table? Sb, In, Cl, Se, Se
1 Answer
Apr 30, 2017
In < Sb < Se < Cl
Explanation:
Electronegativity increases going across (to the right) and up the periodic table.
Cl is the furthest to the top right so it has the highest electronegativity. In is the furthest to the bottom and left so it has the lowest electronegativity. Sb is just to the right of In on the same row so it has slightly higher electronegativity.
Se is farther right and higher up than Sb but lower and farther left than Cl, so its electronegativity is between the two.