How would you calculate the surface tension of a 2% (w/v) solution of a wetting agent that has a density of 1.008 g/mL and that rises 6.6 cm in a capillary tube having an inside radius of 0.2 mm?
1 Answer
The surface tension is
Explanation:
One method to measure the surface tension of a liquid is to measure the height the liquid rises in a capillary tube.
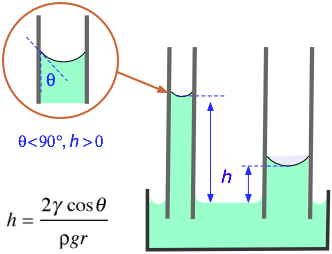
The formula is
#color(blue)(|bar(ul(color(white)(a/a) γ = "rhρg"/"2cosθ" color(white)(a/a)|)))" "#
where
For pure water and clean glass, the contact angle is nearly zero.
If
#color(blue)(|bar(ul(color(white)(a/a) γ = "rhρg"/2 color(white)(a/a)|)))" "#
In your problem,
For comparison, the surface tension of pure water at 20 °C is

