How would you draw a six-carbon alkyne that can exist as a diastereomer?
1 Answer
Dec 21, 2015
A six carbon alkyne that can exist as a diastereomer is 1-ethynyl-2-methylcyclopropane.
Explanation:
One way for a compound to have diastereomers is to have two chiral centres.
The only way we can do this with a six-carbon alkyne is to have a cyclopropane ring.
Then we can add an ethynyl and a methyl group to generate two chiral centres.
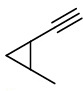
1-ethynyl-2-methylcyclopropane
Since there are two chiral centres and no plane of symmetry, there are
Their structures are
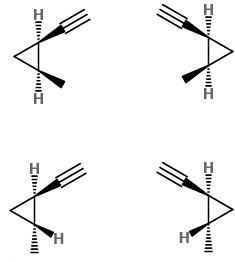
Any isomer in one row is a diastereomer of the two isomers in the other row.
Isomers in the same row are a pair of enantiomers.

