How would you explain the collision theory?
1 Answer
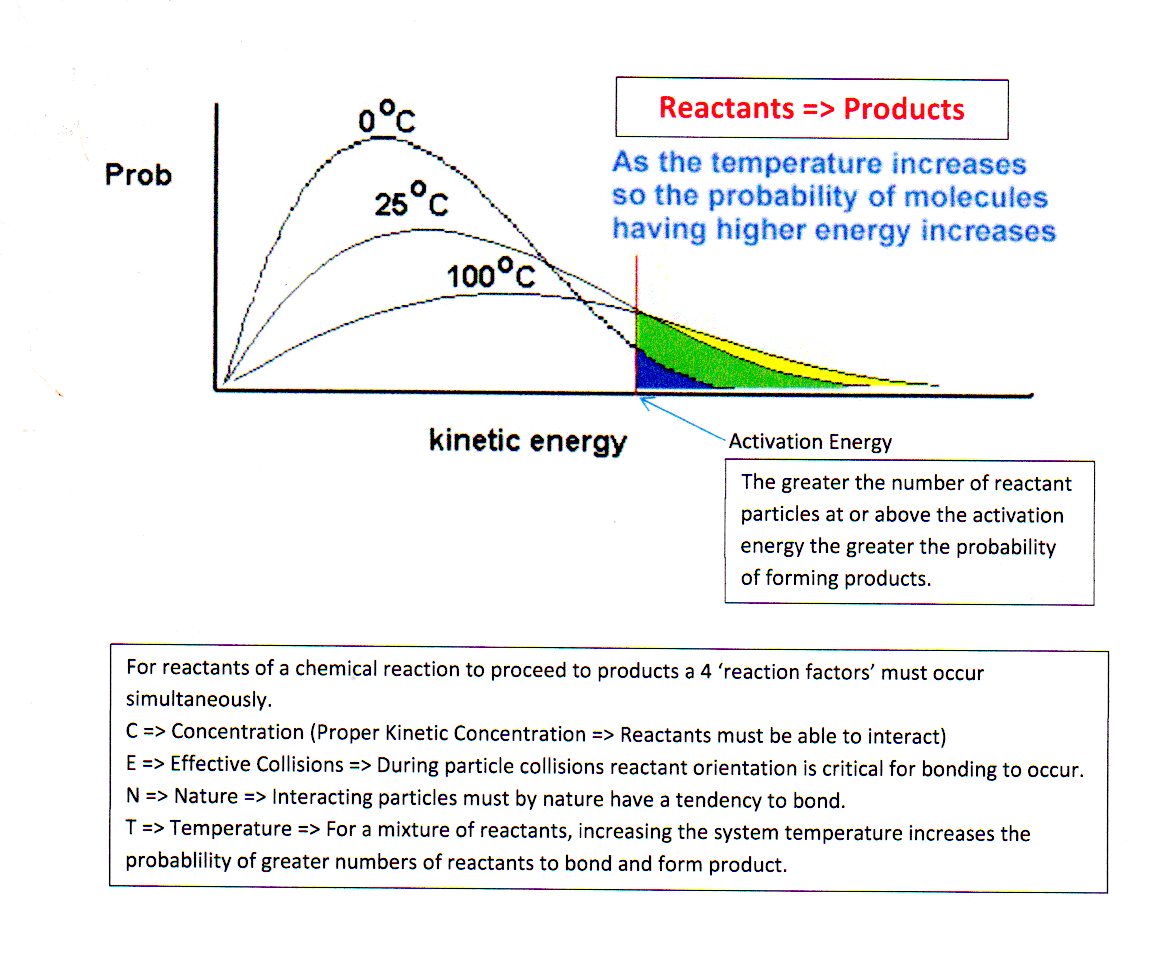
For a reaction to go to completion, four events must occur simultaneously. These include => C.E.N.T.
1. (C) an effective kinetic 'Concentration' of reactants. A + B => Products, A must be able to find B.
2. (E) particle interaction by 'Effective Collisions' . Orientation of collisions is everything.
3. (N) reactants that by 'Nature' will react.
4. (T) increase 'Temperature' of the reaction mixture so that the greatest possible number of particles will have sufficient energy (Activation Energy) to undergo reaction and proceed to products.
Explanation:
The embodiment of The Collision Theory is the simultaneous occurrence of the 4 events listed in the introduction answer.
A good illustration of where these events occur in the process of chemical reactions can be demonstrated using a Maxwell-Boltzman Distribution diagram of particle energy distributions. Figure 1 is such an illustration for reactants at various temperatures.
Figure 1 - Energy Distribution of Particles in a Finite Space
Beyond these fundamental postulates of the Collision Theory, one can find further support of the nature of the chemical reaction process by reviewing 'Transition State Theory' (i.e., Transition State Diagrams) and the 'Kinetics of Chemical Reactions' as defined by the Arrhenius Equation.

