How would you know if a cyclic organic molecule is planar?
1 Answer
When you look at the structure of a six-membered ring, if all the molecular geometries around each atom in the ring are
It's not always the case for rings of other sizes, but for six-membered rings, it usually is true.
...But if all bonds have 100% restricted rotation AND are restricted to be in one plane, then it's even more likely that it IS planar (even if one atom appears to not be
With five-membered rings, having one heteroatom in addition to four
Here are a few examples of planar and non-planar six-membered rings.
Notice how the ring gets more planar with an increasing number of
For five-membered rings, it's harder to find examples, but here are some examples that may contain heteroatoms:
For rings with more than six members, it becomes less likely that all
Its actual structure is:
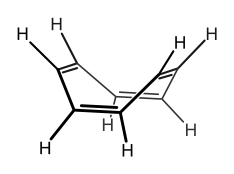
So how would you actually know for sure? Do some X-ray crystallography or X-ray diffraction and determine the structure.

