How would you show the bonding of Hydrogen and Oxygen using Lewis Dot diagrams?
1 Answer
Mar 7, 2017
There are 2 valence electrons from the hydrogen atoms, and 6 valence electrons from the oxygen atom..........
Explanation:
And thus we have to distribute 8 electrons in the Lewis dot diagram.
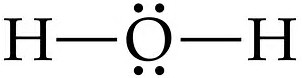
Of course, the electronic geometry is tetrahedral that leads to
Note that I distinguish between electronic geometry, and molecular geometry.

