-ide and -ate, how do you know which one to use?
1 Answer
-ide: used when the anion is monoatomic.
-ate: used when the anion is polyatomic (but still depends on the oxidation state of the ion)
Explanation:
In naming ionic compounds, the name of the metal cation (positively charged) usually goes first followed by the name of the nonmetal anion (negatively charged).
The suffix -ide is only used if the nonmetal anion is monoatomic (meaning one atom).
e.g. chloride (
Notable exceptions to the above rule are the hydroxide (
The use of the suffix -ate, on the other hand, is not that simple. The metal anion must be (1) polyatomic (meaning many atoms) and (2) must satisfy the required oxidation state.
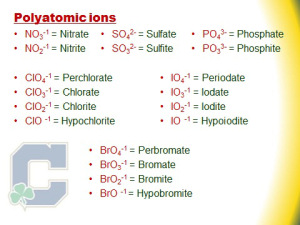
As seen in the image above suggests, the use of the suffix -ate versus the use of the suffix -ite is self-evident.

