If a radioactive Isotope has a half life of 30 days then how long will it take 1000 grams to decay to 120 grams?
1 Answer
Jul 3, 2014
120 days.
Explanation:
Half-life (t½) is the amount of time required for a quantity to fall to half its value as measured at the beginning of the time period.
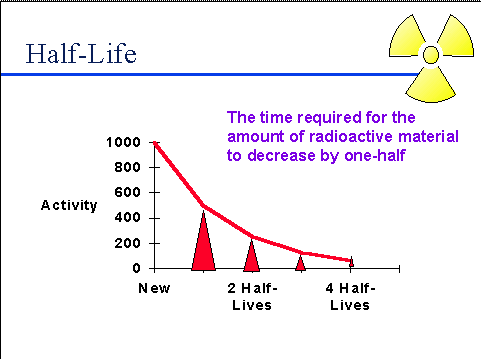
In this question (t½) of isotope is 30 days, which means that after 30 days half of the sample would have decayed and half would be left as it is.
After 30 days ( first half life) 120 /2 = 60 g decays and 60 g remains left.
After another 30 days ( two half lives or 60 days) 60 /2 = 30 g decays and 30 g remains left .
After another 30 days ( three half lives or 90 days) 30 /2 = 15 g decays and 15 g remains left.
After another 30 days ( four half lives or 120 days) 15 /2 = 7.5 g decays and 7.5 g remains left.
after four half lives or 120 days , 7.5 g of the isotope will be left.

