If both double bond and triple bond exist while naming a compound according to IUPAC system, to whom should i give importance first ?
1 Answer
Here is my understanding of the rules.
Explanation:
-
Choose the longest continuous chain of carbon atoms, whether or not it contains multiple bonds.
-
If there is a choice, choose the chain with more multiple bonds.
-
Number from the end of a chain that is closest to a multiple bond.
-
If there is a choice, the double bond gets priority.
-
"Ene" comes before "yne" in the name, no matter what the locants (numbers) are.
Example 1
Consider
The correct name is pent-1-en-4-yne.
Example 2
Example 3
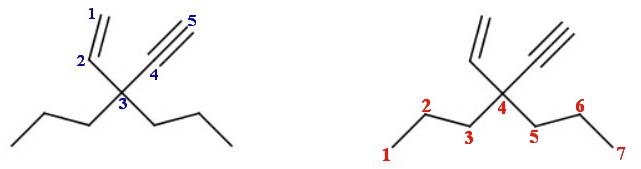
Under the "old" IUPAC rules, the above compound would b
3,3-dipropylpent-1-en-4-yne.
Under the "new" rules, the correct IUPAC name is 4-ethenyl-4-ethynylheptane.

