If carbon 14 has a half life of 5,730 years and a sample contains 70 mg originally, how much is left after 17,190 years?
1 Answer
Jul 3, 2014
Half-life (t½) is the amount of time required for a quantity to fall to half its value as measured at the beginning of the time period.
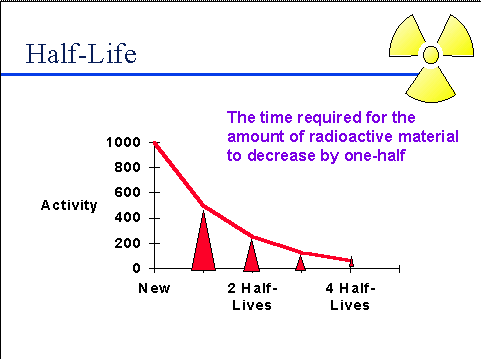
In this question (t½) of C-14 is 5730 years, which means that after 5730 years half of the sample would have decayed and half would be left as it is.
After 5730 years ( first half life) 70 /2 = 35 mg decays and 35 g remains left.
After another 5730 years ( two half lives or 11460 years) 35 /2 = 17.5mg decays and 17.5 g remains left .
After another 5730 years ( three half lives or 17190 years) 17.5 /2 = 8.75mg decays and 8.75g remains left.
after three half lives or 17190 years, 8.75 g of C-14 will be left.

