If excess of #AgNO_"3"# solution is added to 100 ml of 0.024 M solution of dichlorobis (ethylene diamine) cobalt (III) chloride, how many moles of #AgCl# will be precipitated??
1 Answer
Well, this is just a regular limiting reactants problem, with more complicated compounds. The only hurdle is figuring out what the dichlorobis(ethylenediamine)cobalt(III) charge is.
Dichlorobis(ethylenediamine)cobalt(III) chloride has:
- two chloro ligands (
#"Cl"^(-)# ) in the inner coordination sphere. - two ethylenediamine ligands (
#"H"_2"N"-"CH"_2-"CH"_2-"NH"_2# ) in the inner coordination sphere. - a chloride (
#"Cl"^(-)# ) ligand coordinated on the outer coordination sphere. - cobalt(III), which has an oxidation state of... what is
#"III"# in english?
The oxidation states add up as:
#overbrace(2 xx (-1))^"dichloro" + overbrace(2 xx 0)^("bis"("en")) + overbrace((+3))^"cobalt(III)" = (+1)#
Thus, it is written as...
#overbrace(["CoCl"_2("en")_2])^(+1)"Cl"#
The cis form is shown below.
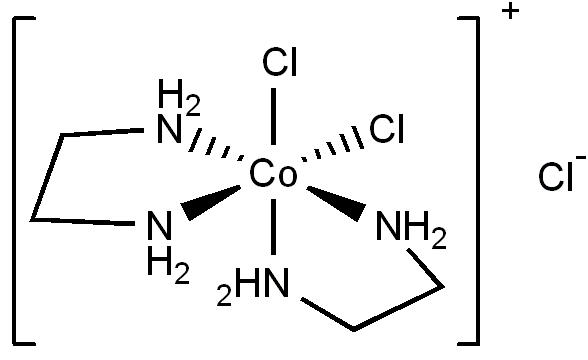
The ethylenediamine ligands are bidentate,
So, the reaction is probably going to be an outer-sphere, double-replacement reaction:
#"AgNO"_3(aq) + ["CoCl"_2("en")_2]"Cl"(aq) -> "AgCl"(s) + ["CoCl"_2("en")_2]"NO"_3(aq)#
The complex is clearly the limiting reactant, so...
#"0.024 mol/L" xx "0.100 L" = "0.0024 mols complex"#
The complex is

