In a #0.70 M# solution of benzoic acid, what percentage of the molecules are ionized (CHEMISTRY 1B problem)?
Benzoic acid is a weak acid that has antimicrobial properties. Its sodium salt, sodium benzoate, is a preservative found in foods, medications, and personal hygiene products. Benzoic acid dissociates in water:
#C_6H_5COOH⇌C_6H_5COO^−+H^+#
The #pKa# of this reaction is #4.2# . In a #0.70 M# solution of benzoic acid, what percentage of the molecules are ionized?
Express the percentage numerically using two significant figures.
- Work
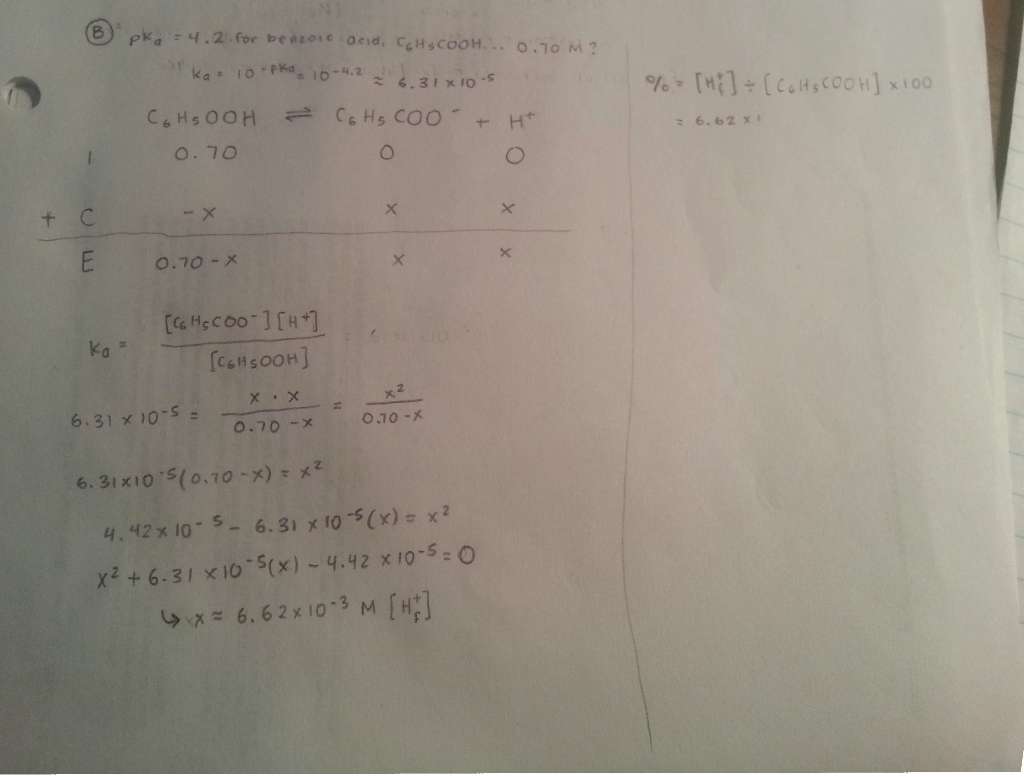
It's incomplete, but I fulfilled the formula, and got (below)...
- Answer result
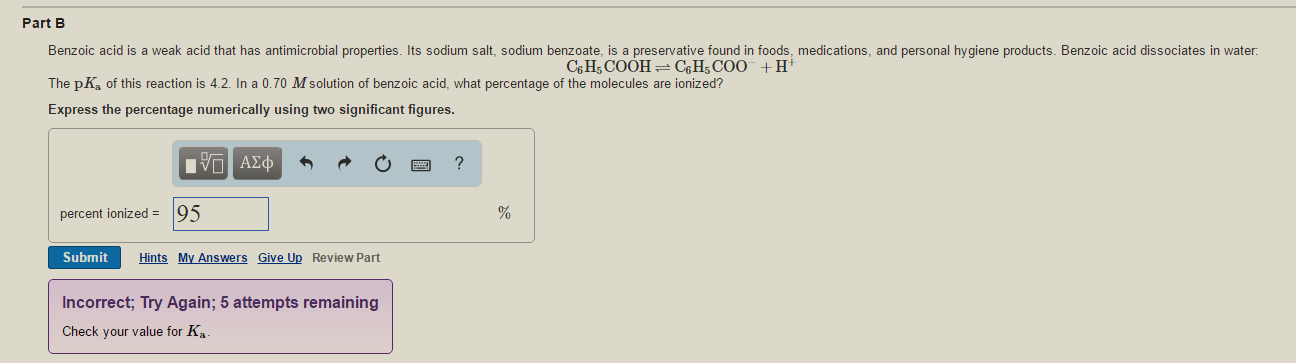
Benzoic acid is a weak acid that has antimicrobial properties. Its sodium salt, sodium benzoate, is a preservative found in foods, medications, and personal hygiene products. Benzoic acid dissociates in water:
The
Express the percentage numerically using two significant figures.
- Work

It's incomplete, but I fulfilled the formula, and got (below)... - Answer result

1 Answer
Mar 14, 2017
( explanation under construction by question owner )
Explanation:

