In a Stock system name such as iron (III) sulfate, what do the Roman numeral tells us?
1 Answer
The Roman numerals indicate the ionic charge of the cation. In this case, it is an ionic charge of
Explanation:
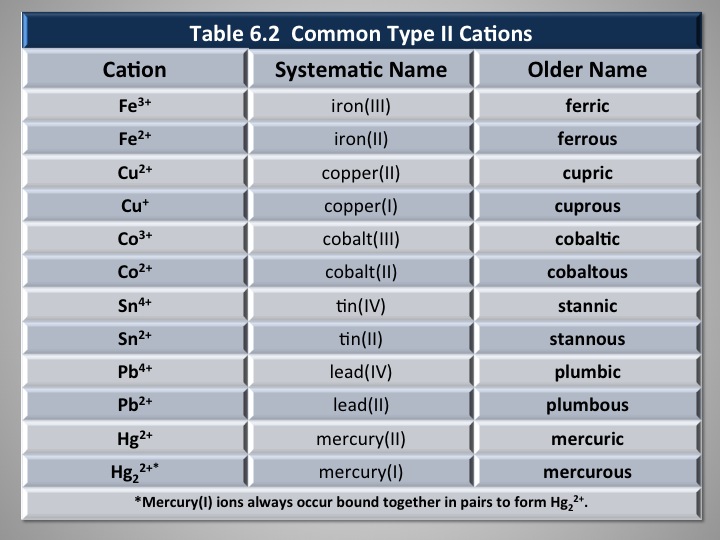
Transition metals are notorious for having multiple ionic charges. Different from being an isotope - an atom that has a different number of neutrons - elements that have Roman numerals right after their chemical name indicate the specific charge of that element.
For example, iron (III) sulfate:

Another composition of iron is iron (II). If reacting with sulfate, it will yield

There are many other atoms that behave this way - having multiple ionic charges.
This video discusses additional examples of how to use the stock system for naming compounds.
Hope this helps :)


