In carbohydrates, if there is more than 1 asymmetrical carbon atom, how do we determine the D and L-forms?
1 Answer
You look at the stereochemistry of the highest-numbered chiral centre in a Fischer projection.
Explanation:
If the
If the
For example, the Fischer projection of D-glucose is
 www.chem.wisc.edu
www.chem.wisc.edu
(from www.chem.wisc.edu)
This is a D-sugar because the
Do not be fooled into thinking that to get the structure of L-glucose, you just invert the configuration at
 metabolomics.jp
metabolomics.jp
(from metabolomics.jp)
This is an L-sugar, but it is L-idose.
To get the structure of L-glucose, you must invert the configuration of every chiral centre.
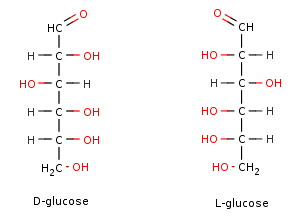 DLGlucose
DLGlucose
(from www.easyochem.com)
D-Glucose and L-glucose are enantiomers — nonsuperimposable mirror images of each other.

