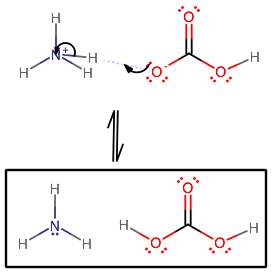
In the following acid-base reaction, how would you identify the acid, base, and their conjugate acids and bases: NH4+ + HCO3- --> NH3 + H2CO3?
1 Answer
Nov 20, 2015
A useful way of thinking about this is:
- Seeing which ion donates a proton (as a Brønsted-Lowry acid) by accepting a pair of electrons (as a Lewis acid)
- Seeing which ion accepts the proton (as a Brønsted-Lowry base) by donating a pair of electrons (as a Lewis base).
Another way to say it is in general, an acid has a proton it can donate, and a base wants a proton.
The change from
The change from
Overall, we have:
- Proton donor (Brønsted-Lowry acid)
- Electron acceptor (Lewis acid)
- Proton acceptor (Brønsted-Lowry base)
- Electron donor (Lewis base)
- Proton acceptor (Brønsted-Lowry base)
- Electron donor (Lewis base)
- Proton donor (Brønsted-Lowry acid)
- Electron acceptor (Lewis acid)
Visually, this is how it is represented: