In the following reactions, ZnO is respectively acting as a /an ?
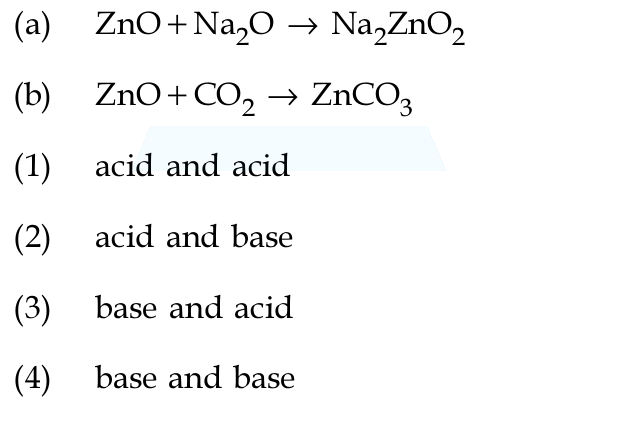
1 Answer
Mar 28, 2018
"Na"_2"O"(aq) -> 2"Na"^(+)(aq) + "O"^(2-)(aq) ,
since
The second reaction is the reverse of a carbonate decomposition. Also,
In fact,

