Is a triple bond always represented in 180° in bond line notations?
1 Answer
Oct 29, 2014
Yes, a triple bond is almost always represented as a straight line in bond line notation.
Each of the alkyne carbon atoms is sp hybridized, with bond angles of 180°.
The two alkyne C atoms and the atoms directly attached to them are all in a straight line.
You can see this see this in the bond line structure for hex-3-yne.

The only exceptions are when the C≡C bond is part of a ring structure and the bonds have no choice except to be bent.
Such molecules have bond angle strain. This make them less stable than acyclic alkynes.
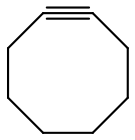
One example is cyclooctyne.
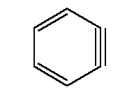
Another is the highly reactive benzyne molecule.