Is #"Na"_2"SO"_4"# soluble in water?
3 Answers
Feb 27, 2018
My word...
Explanation:
Most alkali metal salts are soluble in aqueous solution. Sulfates TEND to be soluble (there are some exceptions that have to be LEARNED).
This site quotes an aqueous solubility of
Feb 27, 2018
Yes.
Explanation:
If you consult a table of solubility rules, like the one below, you will see that sodium sulfate

Yes,
Explanation:
To figure out whether a compound is soluble in water, refer to the following list of solubility rules. This set of rules is very important and should be memorized.
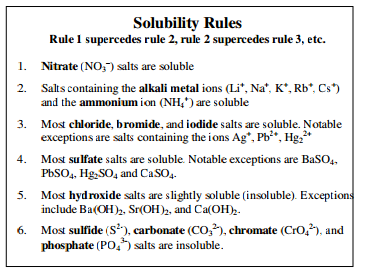
Higher number rules supersede.




