Is there any organic compound that can rebond?
1 Answer
When you say "rebond", I can think of these ways from inorganic and organic chemistry:
Inorganic/Organic Rearrangements
- Bond hapticity being adjusted so that the total valence electron count on a transition metal complex is kept the same.
Organic Rearrangements
- Ring Expansion/Contraction in the presence of a carbocation.
- Alkene rearrangements due to light- or heat-induced catalysis, or olefin metathesis.
Since you should be taught about olefin metathesis in class eventually, I'll leave that to your professor.
DISCLAIMER: LONG ANSWER!
INORGANIC REARRANGEMENTS
Bond Hapticity Aajustment -
Hapticity (
For this compound,
- Each neutral
#"CO"# contributes two valence electrons, for a total of#\mathbf(10)# . - The neutral
#eta^1# -allyl ligand, which bonds via one atom, contributes#\mathbf(1)# valence electron. - Manganese is therefore
#"Mn"^(0)# , and has#\mathbf(7)# valence electrons.
Thus, this compound is a
Upon heating or subjecting this compound to UV-light, one
But, the
Now, the allyl (an organic delocalized
There are plenty more examples in Transition Metal chemistry, but that's one I could think of off the top of my head.
ORGANIC REARRANGEMENTS
These are much more interesting to describe, and usually happen with conjugated
There are quite a few variations, but as some examples, I'll look at:
- a ring expansion/contraction (you should have seen this before)
- a disrotatory ring closure (thermal catalysis)
- a conrotatory electrocyclic ring-opening (thermal catalysis)
Ring Expansion/Contraction -
Expansion usually occurs when you have a formed cationic carbon adjacent to a small ring (4/5 members) that can be stabilized by expanding intramolecularly.
(Ring contractions will occur for 7/8 membered rings.)
The cationic carbon can appear when you add a strong acid (e.g.
The major product is the expanded ring.
Disrotatory Electrocyclic Ring Closure
This usually occurs in straight-chained conjugated
A conrotatory process means the end-
(The HOMO contains matching signs on orbital pairs, going
So, a disrotatory process is when they rotate towards each other (say, CW vs. CCW). This example is of 2,4,6-octatriene.
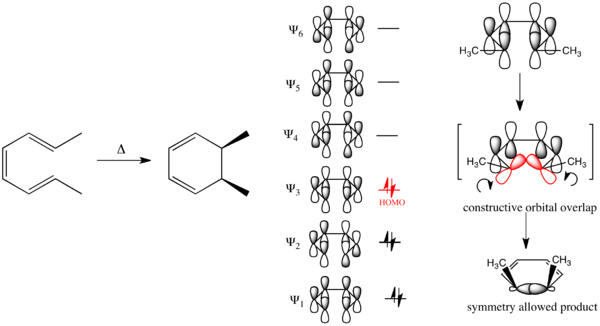
Because these orbitals rotated towards each other, the stereochemistry of the final product's methyl groups is cis. The arrow-pushing mechanism would look like this:
Conrotatory Electrocyclic Ring-Opening
A ring-opening tends to occur with small
Then, the end-
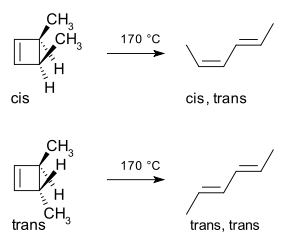
In the image, both end-orbitals rotate CCW, generating the HOMO of the system, which has matching signs on orbital pairs, as in,
The conrotatory process resulted in the methyl groups facing in the same direction, generating the cis,trans-2,4-hexadiene isomer.

