If #K_p = 2.4 * 10^(-3)# for the reaction below, then what is #K_c# ?
1 Answer
Explanation:
Your tool of choice here will be the equation
#color(blue)(ul(color(black)(K_p = K_c * (RT)^(Deltan))))#
Here
#K_p# is the equilibrium constant in terms of partial pressures#K_c# is the equilibrium constant in terms of concentrations#R# is the universal gas constant, equal to#0.0821 quad ("atm" * "L")/("mol" * "K")# #T# is the absolute temperature at which the reaction takes place#Deltan# is the difference between the number of moles of gaseous products and the number of moles of gaseous reactants
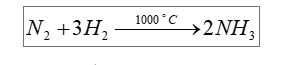
Now, your reaction takes place at
#T = 1000^@"C" + 273.15 = "1273.15 K"#
Notice that for every
This means that you have
#Deltan = color(white)(overbrace(color(black)(" 2 "))^(color(blue)("moles of ammonia")) " "color(black)(-)" " overbrace(color(black)((" 1 + 3 ")))^(color(blue)("moles of reactants"))#
#Deltan = - 2#
Rearrange the equation to solve for
#K_c = K_p/((RT)^(Deltan)#
Plug in your values to find--since you didn't provide any units for
#K_c = (2.4 * 10^(-3))/(0.0821 * 1273.15)^(-2) = color(darkgreen)(ul(color(black)(26)))#
The answer is rounded to two sig figs, the number of sig figs you have for

