Liquid nitrogen is used by physicians to burn moles off of skin. Liquid nitrogen boils at 77 K. What is its boiling point in °C?
1 Answer
Explanation:
The problem wants you to use the conversion factor that takes you from Kelvin to degree Celsius to calculate the boiling point of liquid nitrogen in degrees Celsius.
As you know, the Celsius and Kelvin temperature scales differ from each other in the placement of the zero mark. In other words, an increase of
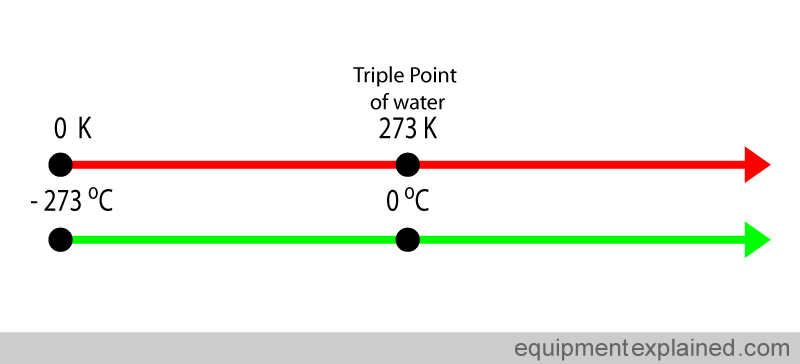
More specifically, the zero mark on a Celsius scale is set on
The conversion factor that takes you from degrees Celsius to Kelvin and vice versa looks like this
#color(purple)(|bar(ul(color(white)(a/a)color(black)(t["K"] = t[""^@"C"] + 273.15)color(white)(a/a)|)))#
So, in order to go from Kelvin to degrees Celsius, you must subtract
You will thus have
#t[""^@"C"] = "77 K" - 273.15 = -196.15^@"C"#
Rounded to the number of decimal places of
#t[""^@"C"] = color(green)(|bar(ul(color(white)(a/a)color(black)(-196^@"C")color(white)(a/a)|)))#

