Meaning- "Sigma bonds can occur between any kind of atomic orbitals; the only requirement is that the atomic orbital overlap happens directly between the nuclei of atoms."?
1 Answer
Jun 12, 2017
It is saying that the bonding electrons must be directly between the nuclei of the bonding atoms.
Explanation:
Sigma (σ) bonds are formed by head-on overlapping of atomic orbitals.
 upload.wikimedia.org
upload.wikimedia.org
This puts most of the electron density along the internuclear axis between the two nuclei.
Here are some examples of σ bonds,
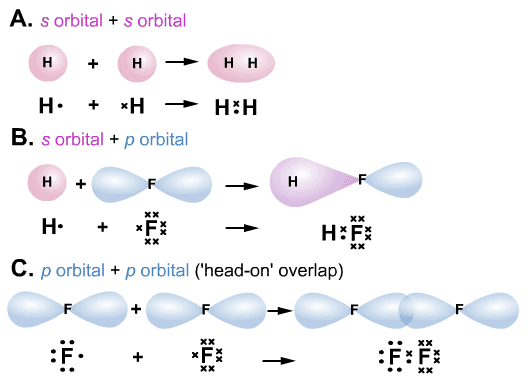 leaving-cert-notes.weebly.com
leaving-cert-notes.weebly.com
Hydrogen has an
Ethane
(From Google Sites)
Ethane has an
You can also have
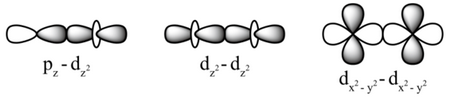 d sigma
d sigma
(Adapted from Boundless)
In all of these σ bonds, the bonding electrons are on the internuclear axis between the two nuclei.

