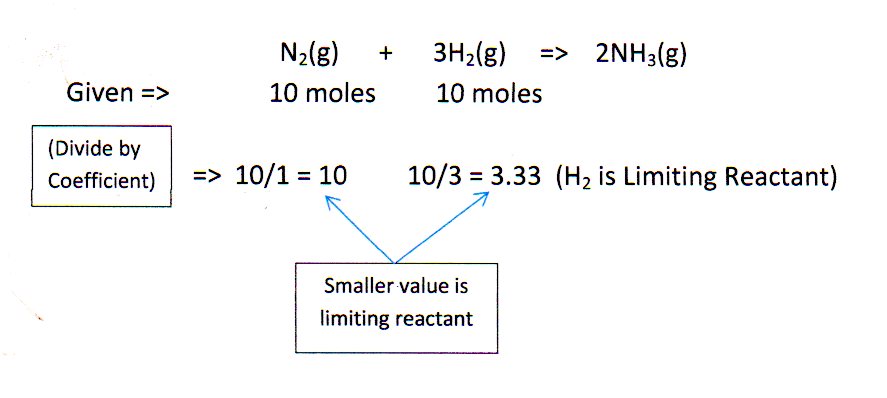
#N_2 + 3H_2 -> 2NH_3#. What is the limiting reactant if you start the reaction with 10.0 moles of each reactant?
2 Answers
May 22, 2017
Explanation:
The reaction equation says that three moles of
May 22, 2017
Explanation:
A quick way to determine limiting reagent (or, reactant) is to convert all data dimensions to moles and divide each by the respective coefficient in the balanced Equation. The smaller number will be the limiting reactant.