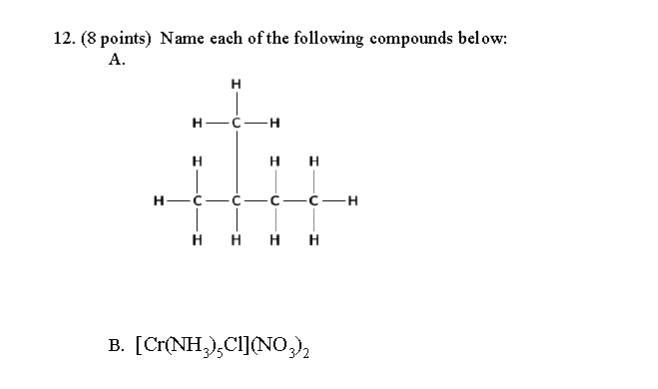
Name each of the following compounds?
1 Answer
The first one is a kind of hydrocarbon. When we identify the main chain, we can create the parent name to the IUPAC name.
The main chain has
Therefore, this compound is some sort of butane-based compound, resembling this:
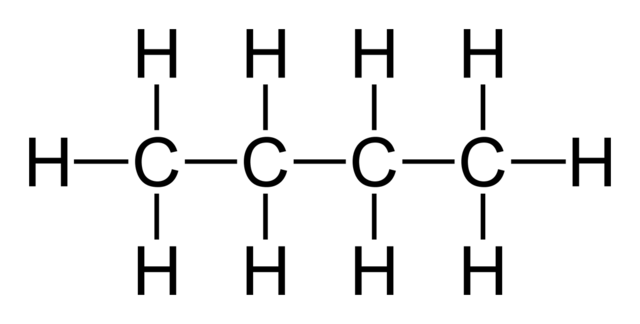
If we number the main chain carbons (in accordance with IUPAC Nomenclature rules) so that each sidechain is the lowest number possible
The sidechain is
So, the compound is called
#bb(2-"methylbutane")# .
In this inorganic transition metal complex, we take a somewhat different approach and look at the charge of the outer ligand (on the second coordination sphere).
#["Cr"("NH"_3)_5"Cl"]stackrel("Nitrate")overbrace(("NO"_3)_2)#
Each nitrate ion (
#stackrel("+2 charge")overbrace(["Cr"("NH"_3)_5"Cl"])stackrel("-1 charge each")overbrace(("NO"_3)_2)# This polyatomic ion's name is simply going to be appended, like when naming any ionic compound with two ions (such as silver nitrate, or calcium nitrate).
The chloro ligand (
These ligands in the inner coordination sphere will have prefixes indicating how many of each there are, except if there is
#1# .Also, if the prefix ends in a vowel and the ligand starts with a vowel, the double vowel is omitted. So, "pent" will go in front of "ammine".
The remaining charge has to balance out to give
#x + (-1) = +2#
#=> x = color(blue)(+3)#
#=> [stackrel(+3)"Cr"stackrel(0)(("NH"_3)_5)stackrel(-1)"Cl"]^(2+)#
So, we have
Put that all together in all lowercase without a space (except for the appended anion name), in alphabetical order of ligand name:
#stackrel("alphabetical order of ligand name")overbrace("prefix" + "ligand" + . . . + "prefix" + "ligand") + "metal"("oxid. number") + "anion name"#
#=> "pentammine" + "chloro" + "chromium(III)" + "nitrate"#
#=> color(blue)("pentamminechlorochromium(III) nitrate")#
And since only one ligand differs in the inner coordination sphere, there is no special symmetry that we should account for in the naming.
(No cis/trans, no

