#NH_4OH + HCl(aq) = NH_4Cl(aq) + H_2O(l)# no significant change can be noticed How to make sure that a reaction is really taking place ?
1 Answer
Jul 21, 2017
Can you not use an indicator?
Explanation:
You titrate ammonia with hydrochloric acid......
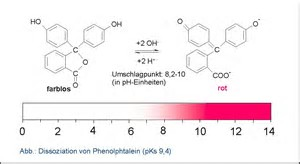
At the stoichiometric endpoint, the pink dissipates to colourless. And there are other indicators to use if you don't like pink.

