On combustion, benzoic acid releases 26.38 KJ/g. The bomb calorimeter contained 1.000 g of benzoic acid. The temperature of the calorimeter rises from 24.32 degrees C to 27.05 degrees C?
#a)# What is #DeltaT# of the calorimeter?
#b)# How much heat is absorbed by the bomb calorimeter?
#c)# What is the heat capacity, #C_(cal)# , of the calorimeter, in #"kJ/"^@ "C"# ?
2 Answers
see below
Explanation:
The absorbed heat is 26,38 KJ. But a calorimeter is made from a contenitor that is heated (
A bomb calorimeter is a rigid container that looks as follows:
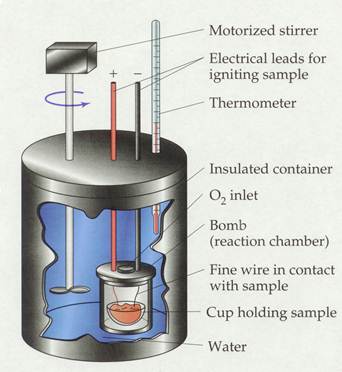
The benzoic acid is placed into the sample cup inside the bomb, and about
The change in temperature of the water IS the change in temperature of the calorimeter:
#DeltaT = 27.05^@ "C" - 24.32^@ "C" = ???#
The heat absorbed by the calorimeter is the heat released by the reaction, by construction. The massive amount of water will ensure excellent insulation and thus conservation of energy.
Hence, the heat absorbed is
#"26.38 kJ"/cancel"g benzoic acid" xx cancel"1.000 g benzoic acid" = color(blue)("26.38 kJ")#
The heat absorbed then gives the heat capacity:
#"26.38 kJ" = C_(cal)DeltaT_(Cal)#
#=> color(blue)(C_(cal) = "26.38 kJ"/(DeltaT_(Cal)) = ??? "kJ/"^@ "C")#
CHALLENGE: Is

