One of the outermost electrons in a strontium atom in the ground state can be described by what sets of four quantum numbers?
1 Answer
Here's what I got.
Explanation:
Your starting point for this problem will be the electron configuration for a neutral strontium atom in its ground state, which looks like this
#"Sr: " 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 color(red)(5)s^2#
As you can see, strontium's outermost electrons are located on the fifth energy level, in the 5s-orbital.
In order to completely describe these two electrons, you need to list the values of the four quantum numbers used to characterize their location and spin.
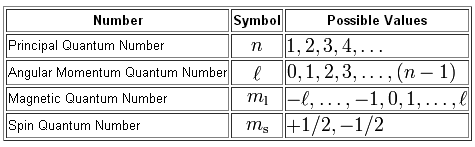
So, you know that the two electrons are located on the fifth energy level, which means that the principal quantum number,
The angular momentum quantum number,
For the s-subshell, the magnetic quantum number,
Finally, the spin quantum number,
Since two electrons occupy the 5s-orbital, one will have
Therefore, the quantum sets that describe the two outermost electrons electrons in a strontium atom will be
#n=5, l=0, m_l = 0, m_s = +1/2#
and
#n=5, l=0, m_l = 0, m_s = -1/2#

