Help with chem question?
A molecule consists of one sulfur atom and two oxygen atoms.
a. Draw a Lewis structure for this molecule that does NOT include an expanded octet. If the structure involves resonance, draw all the resonance structures.
b. Draw a Lewis structure that shows an expanded octet on a central sulfur atom. Use formal charges to determine if the Lewis structure question ‘a’ or question ‘b’ is the more likely Lewis structure.
c. What is the electron geometry and the molecular geometry of this molecule?
d. Identify any sigma bonds and any pi bonds in the molecular structure you drew in question ‘a’. Describe the location of the electron densities for each of these two different types of bonds.
e. Predict whether this molecule will be more attracted to water or hexane (C6H14). Justify your selection.
A molecule consists of one sulfur atom and two oxygen atoms.
a. Draw a Lewis structure for this molecule that does NOT include an expanded octet. If the structure involves resonance, draw all the resonance structures.
b. Draw a Lewis structure that shows an expanded octet on a central sulfur atom. Use formal charges to determine if the Lewis structure question ‘a’ or question ‘b’ is the more likely Lewis structure.
c. What is the electron geometry and the molecular geometry of this molecule?
d. Identify any sigma bonds and any pi bonds in the molecular structure you drew in question ‘a’. Describe the location of the electron densities for each of these two different types of bonds.
e. Predict whether this molecule will be more attracted to water or hexane (C6H14). Justify your selection.
1 Answer
See below.
Explanation:
a.
In this structure, the
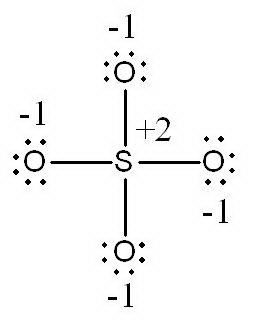
(Adapted from SlidePlayer)
There are no other resonance structures with a "normal octet**.
b.
In this structure, the
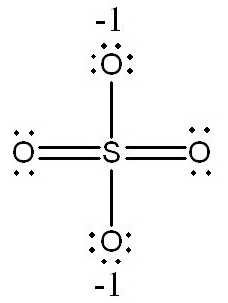
(Adapted from SlidePlayer)
It has fewer formal charges than the structure in a., so it is a better Lewis structure.
c.
There are four electron regions around the
The electron regions include no lone pairs, so the molecular geometry is also tetrahedral.
d.
There are only
The electron density is concentrated between the two nuclei and along the internuclear axis.
The image below shows an
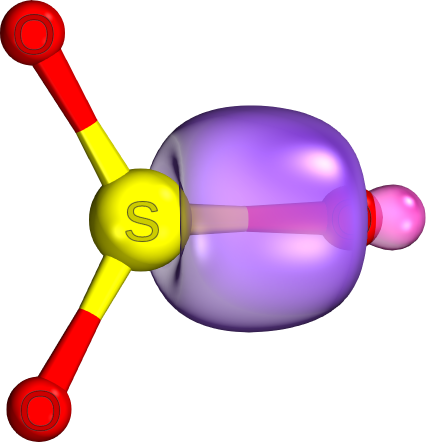
In a pi bond, the electron density is concentrated in two areas between the two nuclei but away from the internuclear axis, and there is a nodal surface between them.
The image below shows both a
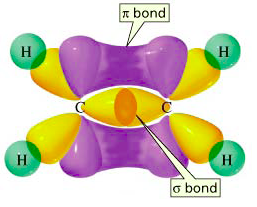
e.

