Suppoose "A" Is any molecule whose mirror image is "B". The " A" Nd "B" Are not superimposed.The mirror image of "B" is "C". The " C" Is superimposed to "A". So what can we say molecule "A" Is chiral r Achiral.????plzzzz help.
1 Answer
I'm not sure I really understand what you're asking, but let's have a go...
Explanation:
Chirality, mirror-images, D/L-configurations and the like are caused by Asymmetrical Carbon Centres. A Carbon atom can make 4 covalent bonds. Their juxtaposition resembles something like the steering wheel of a car:
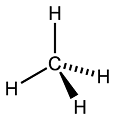
Let's number the H-atoms:
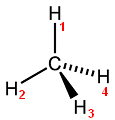
You can see that H-atoms 2,3 and 4 are numbered Counter-clockwise...
If instead we number them Clockwise, then we get the Mirror-image:
These two are super-imposable, because the bonds are all with (identical) Hydrogen atoms. If we replace
But the game changes when the central C-atom binds to 4 groups that are ALL different: Suddenly the structure has lost its symmetry, making superimposition impossible!
But make note of the fact that one asymmetrical C-atom can result in only TWO different molecules ( called Enantiomers ).
So, back to your question: B is the mirror image of A, and they are not super-imposable.
If C is the mirror image of B, then it follows that C must be identical to A. You mentioned yourself that A and C ARE Superimposable....
If A and B are not super-imposable, then A (and consequently B) have at least one Chiral Carbon centre...
Achiral means non-chiral.....
.

