The freezing point depression of potassium ethanoate is greater than that of the other two solutes (camphor and urea) when an equal number of moles of each is used. Can somebody explain this?
In the class we only measured benzoic acid with potassium ethanoate. We did not measure camphor or urea. 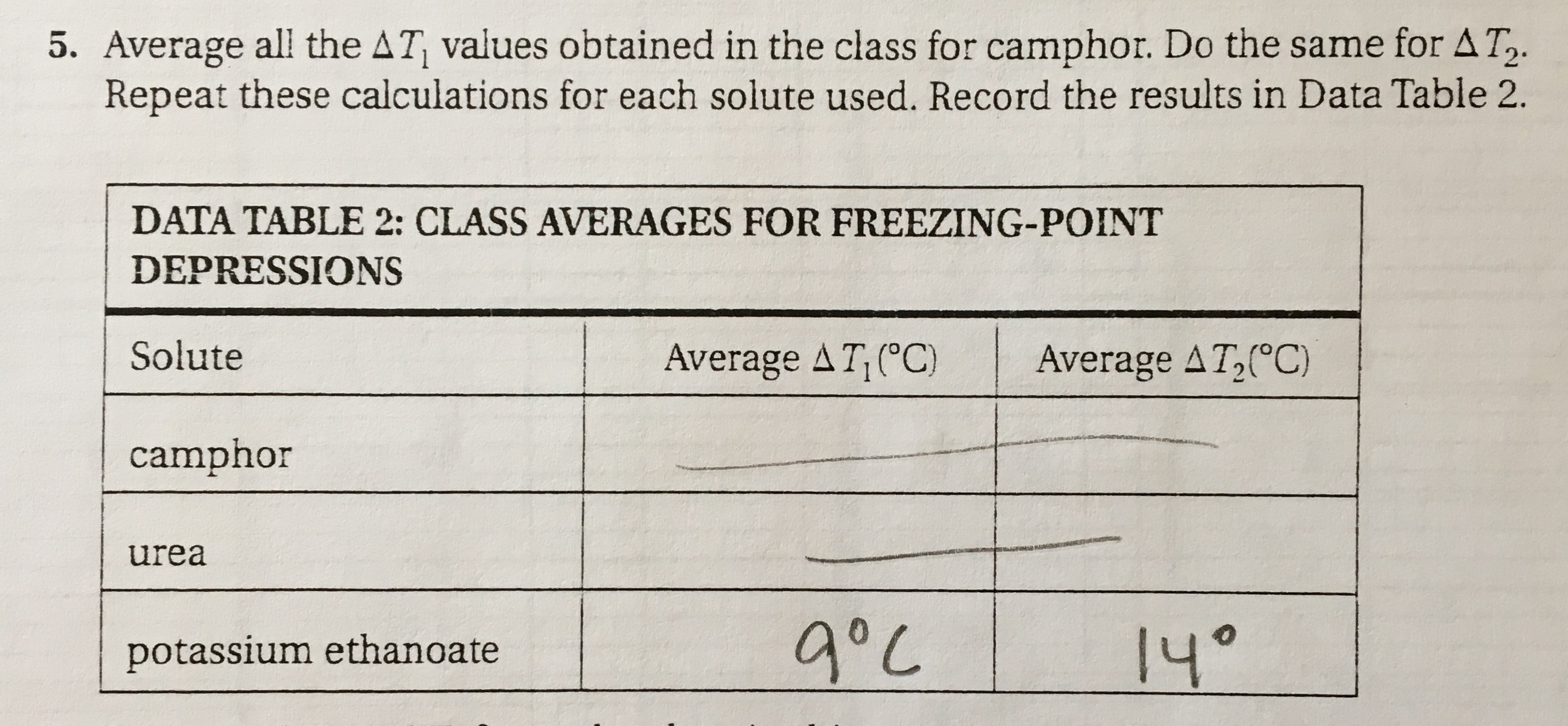
In the class we only measured benzoic acid with potassium ethanoate. We did not measure camphor or urea. 
1 Answer
Apr 8, 2017
The freezing point depression effect is based on the number of particles (colligative properties), not the specific compound.
Explanation:
The freezing point depression effect is based on the number of particles (colligative properties), not the specific compound.
Potassium ethanoate is the only compound in the list that dissociates into two parts, effectively doubling the number of particles in solution per mole of original solute.

