The "pH" at one-half the equivalence point in an acid-base titration was found to be 5.67. What is the value of K_a for this unknown acid?
1 Answer
Explanation:
The idea here is that at the half equivalence point, the
Assuming that you're titrating a weak monoprotic acid
"HA"_ ((aq)) + "OH"_ ((aq))^(-) -> "A"_ ((aq))^(-) + "H"_ 2"O"_ ((l))
So when you're adding equal numbers of moles of weak acid and of strong base, all the moles of the weak will be consumed and you'll be left with
Now, at the half equivalence point, you're adding enough moles of the strong base to neutralize half of the moles of the weak acid present in the solution.
The reaction will consume half of the moles of the weak acid and produce just as many moles of the conjugate base
And so at the half equivalence point, the solution will contain equal numbers of moles of the weak acid and of its conjugate base, which implies that you're now dealing with a buffer solution.
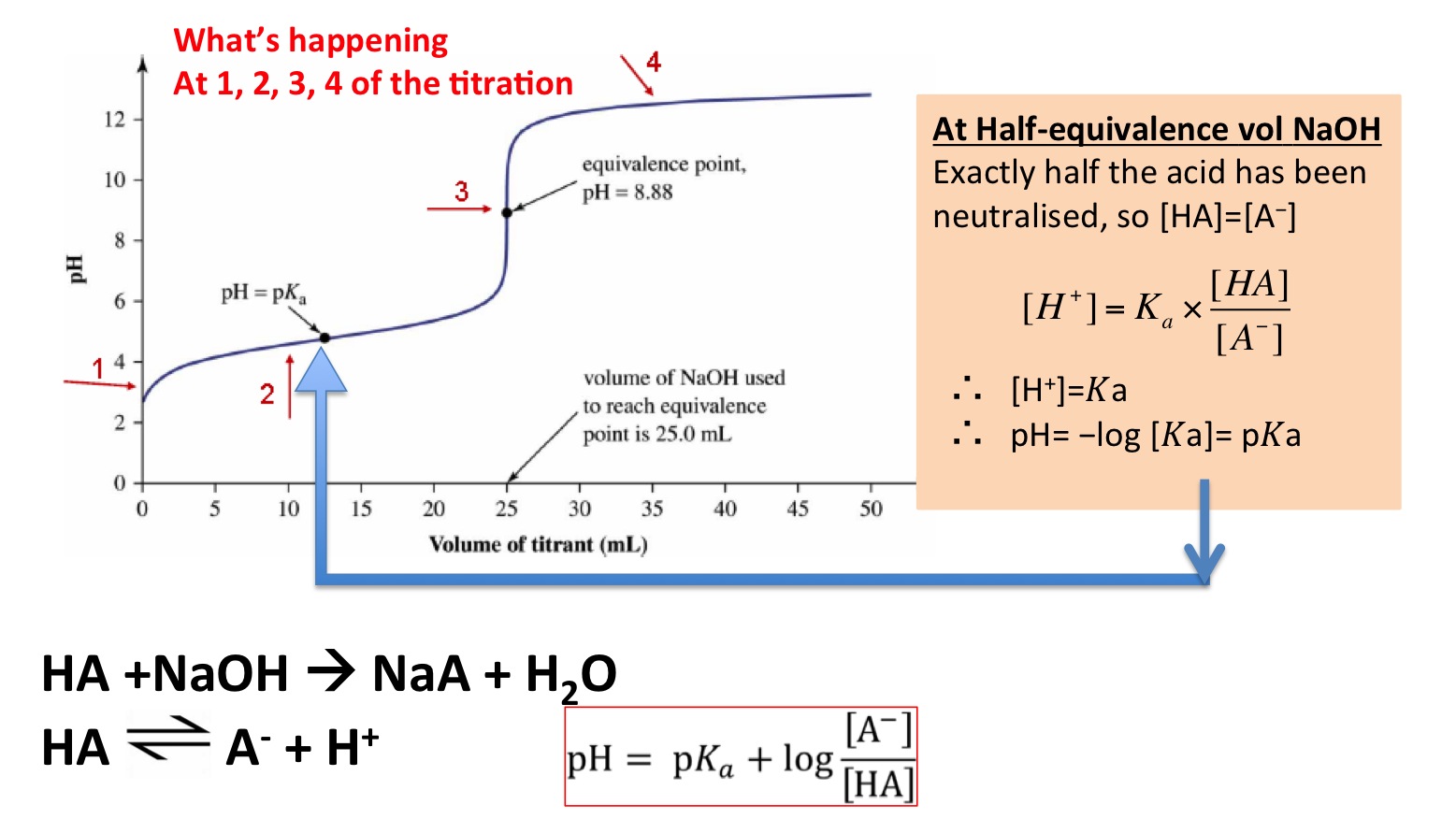 https://secondaryscience4all.wordpress.com/2014/08/10/ph-curves-titrations-and-indicators/
https://secondaryscience4all.wordpress.com/2014/08/10/ph-curves-titrations-and-indicators/
As you know, the
"pH" = "p"K_a + log( (["conjugate base"])/(["weak acid"]))
At the half equivalence point, you have
["HA"] = ["A"^(-)]
which implies that
log( (["HA"])/(["A"^(-)])) = log(1) = 0
Therefore, you can say that at the half-equivalence point, the
color(blue)(ul(color(black)("At the half equivalence point: " -> " pH" = "p"K_a)))
The
"p"K_a = - log(K_a)
which implies that you have
K_a = 10^(-"p"K_a)
At the half equivalence point, you will have
K_a = 10^(-"pH")
Plug in your value to find
K_a = 10^(-5.67) = color(darkgreen)(ul(color(black)(2.1 * 10^(-6))))
The answer is rounded to two sig figs, the number of decimal places you have for the

