The pH of a solution is 8.7, what is the pOH?
1 Answer
Jul 5, 2016
pOH = 5.3
Explanation:
You can answer this question in one of two ways:
- Take the anti-log of the pH to obtain the concentration of
#H^+# ions in solution. After that, use the self-ionization of water formula:

Where
- Subtract the pH from 14 to obtain the pOH.
I'll show you both ways using these equations:
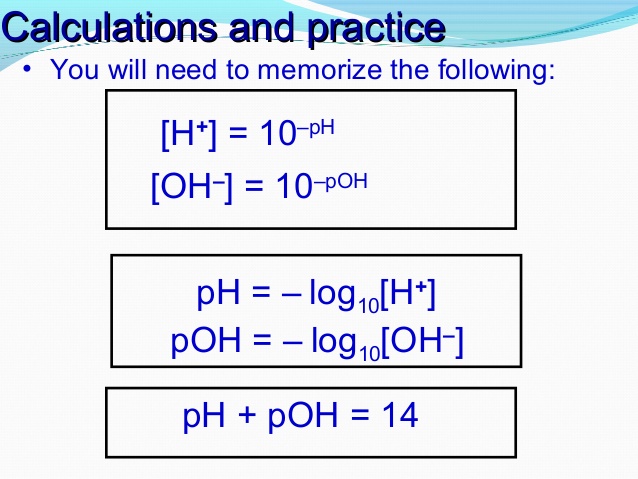
THE PROCESS FOR METHOD 1:
Kw / [H+] = [OH-]
THE PROCESS FOR METHOD 2:

