The reaction #2Na + Cl_2 -> 2NaCl# is an example of which type of reaction?
1 Answer
See explanation.
Explanation:
The reaction you have here
#2"Na"_ ((s)) + "Cl"_ (2(g)) -> 2"NaCl"_ ((s))#
is an example of a synthesis reaction.
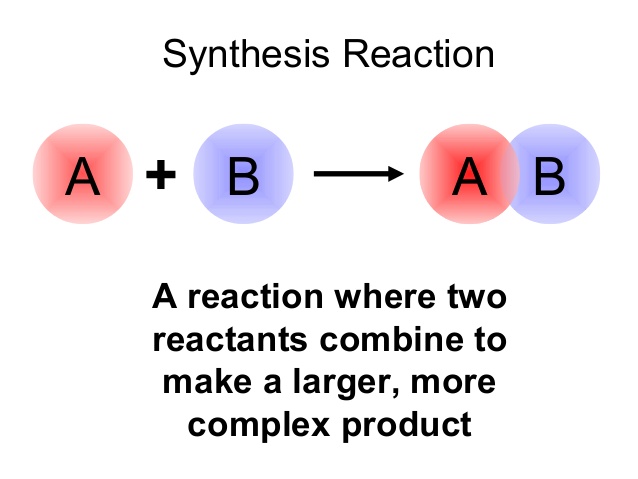
In your case, sodium metal,
You can also say that the reaction given to you is an example of a redox reaction.
#2 stackrel(color(blue)(0))("Na") _ ((s)) + stackrel(color(blue)(0))("Cl") _ (2(g)) -> 2 stackrel(color(blue)(+1))("Na") stackrel(color(blue)(-1))("Cl")_ ((s))#
The oxidation number of sodium increases from
On the other hand, the oxidation number of chlorine decreases from

