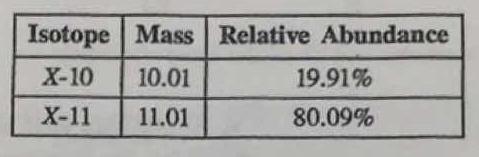
The table below gives information about two isotopes of element X. How would you calculate the average atomic mass of element X?
1 Answer
Feb 1, 2017
Explanation:
Step by step:
Multiply the masses of the isotopes by their respective relative abundances, and then add them together to find the average atomic mass of element X.
Fundamentally:
Let's recall that an isotope is a form of an element with a variable amount of neutron (and therefore varying amount of atomic mass).
So if we multiply the two mass forms of the element with varying neutron by their relative frequencies and then find the sum of the two products, we will acquire the average atomic mass of element X.

