Use half-cell potentials to calculate #K_(eq)# for the reaction below?
#2Cu# (s)#+2H^+# (aq)#\toH_2# (g)
#E°_{Cu\toCu^(2+)}=-0.34# V
My work:
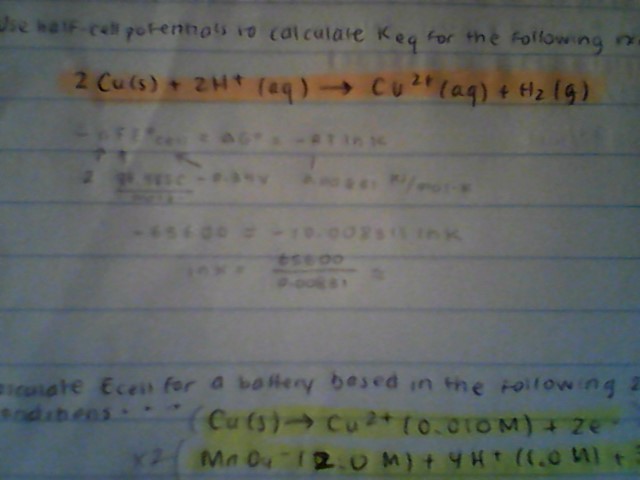
My work:
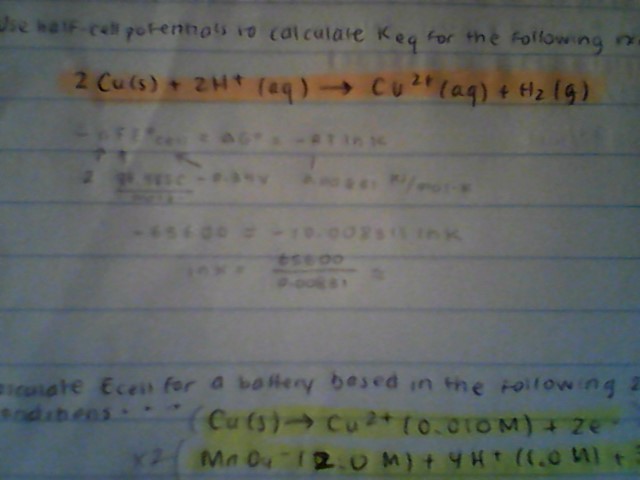
2 Answers
Explanation:
Using
For above reaction:
n = 2 and
Well, for this nonspontaneous reaction...
First of all, let's re-balance the reaction, which should be:
#"Cu"(s) -> "Cu"^(2+)(aq) + 2e^(-)# ,#E_(red)^@ = +"0.34 V"#
#ul(2"H"^(+)(aq) + 2e^(-) -> "H"_2(g))# ,#E_(red)^@ = "0.00 V"#
#2"Cu"(s) + 2"H"^(+)(aq) -> 2"Cu"^(2+)(aq) + "H"_2(g)#
The reduction potential of copper cation is more positive, so copper would rather be reduced.
Hence, this reaction is nonspontaneous as-written if we try our hardest and certainly fail to oxidize copper using
The (nonspontaneous) cell potential is:
#E_(cell)^@#
#= overbrace(E_(red)^@)^"reduction" +overbrace(E_(o x)^@)^"oxidation"# , OR#overbrace(E_("cathode")^@)^"reduction" - overbrace(E_"anode"^@)^"reduction"#
#= "0.00 V" + (-"0.34 V")# , OR#"0.00 V" - (+"0.34 V")#
#= -"0.34 V"#
From this we obtain the positive
#DeltaG^@ = -nFE_(cell)^@#
#= -(2 cancel("mol e"^(-)))/("1 mol Cu") cdot "96485 C/"cancel("mol e"^(-)) cdot (-"0.34 V")#
#=# #"65610 J/mol"#
As a result, since we are at equilibrium,
#cancel(DeltaG)^(0) = DeltaG^@ + RTlncancelQ^K#
and thus, we get the physically impractical equilibrium constant:
#color(blue)(K) = e^(-DeltaG^@//RT)#
#= e^(-(+"65610 J/mol")//("8.314 J/mol"cdot"K" cdot "298 K")#
#= e^(-26.5)#
#= color(blue)(3.16 xx 10^(-12))#


