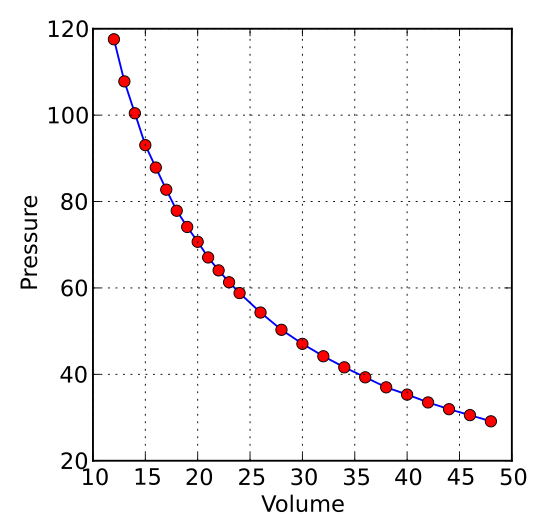
Using Boyle's law why is a graph showing the relationship between pressure and volume not linear?
1 Answer
May 20, 2018
Well, what is
Explanation:
And so
And thus the normal expression of
And when we graph pressure against volume for a given quantity of gas at constant temperature....we gets...