Using the phase diagram for CO2, what phase is carbon dioxide in at -20 C and 1 atm pressure?
1 Answer
Jul 31, 2017
The gas phase
Explanation:
A phase diagram for
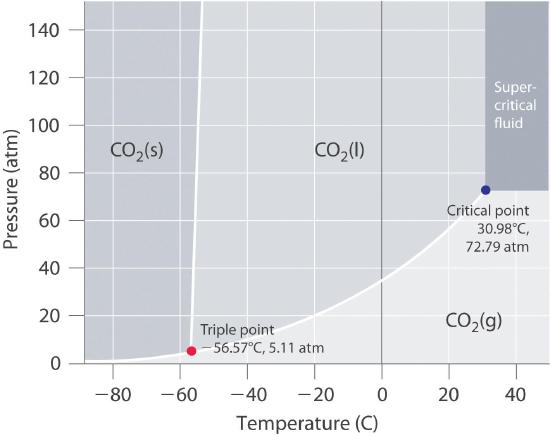 chem.libretexts.org
chem.libretexts.org
What we do is take the vertical line of
Carbon dioxide appears to be in the gaseous state at
