#tildenu = c - R_H / n_2^2# (#tildenu = "wavenumber"#) This equation can be written in the form #y = mx + c# What will the intercept of the line on the #y# axis (at #x = 0#) be, in terms of #n_1#?
1 Answer
The y-intercept is:
#c = R_H/n_1^2#
assuming this is for a fixed starting quantum level
For example, if we obtained frequencies from a hydrogen absorption spectrum based on excitations from an unknown
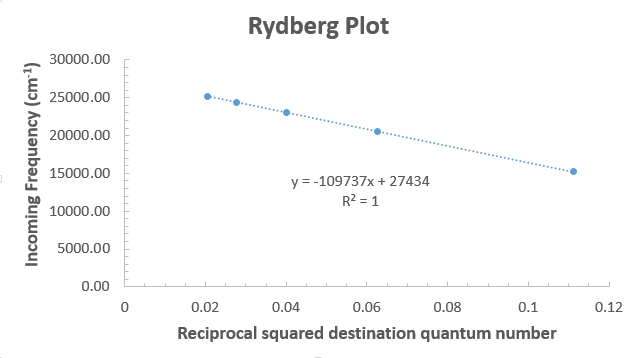
From this, the slope is
Since
#n_1 = sqrt(R_H/c) = sqrt("109737 cm"^(-1)/("27434 cm"^(-1))) = 2.000002 ~~ 2#
So, the starting quantum level was
- Had we guessed
#n_2 = 4,5,6,7,8# , we would get#n_1 = 2.721963# , with an#R^2 = 0.9961# . - Had we guessed
#n_2 = 5,6,7,8,9# , we would get#n_1 = 3.465822# , with an#R^2 = 0.9886# . - Had we guessed
#n_2 = 6,7,8,9,10# , we would get#n_1 = 4.229976# , with an#R^2 = 0.9807# .
Lastly, because the peaks were of very low intensity, it couldn't be that
(You really can tell when you've not correctly guessed the lowest
If you want to plot
#tildenu = c - R_H/(n_2^2)#
you would use
#overbrace(tildenu)^(y) = -R_H(1/n_2^2 - c/(R_H))#
#= overbrace(-R_H)^"slope" cdot overbrace(1/n_2^2)^(x) + overbrace(c)^("y-intercept")# where
#R_H = "109737 cm"^(-1)# is the Rydberg constant.
We compare with the actual equation:
#tildenu = -R_H(1/n_2^2 - 1/n_1^2)#
From this, we see that
#c/R_H = 1/n_1^2#
Therefore, the y-intercept would be:
#color(blue)(c = R_H/n_1^2)#
and the slope would be the ground state energy of hydrogen atom,

