VBT theory predict Hybridization OR derive Hybridization from VSEPR theory VBT explains bonding with respect to VSEPR - it didn't expect Hybridization H2O SP3 due to vespr not vbt expect second why there is no Hybridization in HF for VBT explaination ?
1 Answer
Here's my answer to what I think you are asking.
Explanation:
Valence bond theory
Valence Bond (VB) theory is based on valence electrons. We use it it help determine the structure of a molecule.
We start with the Lewis structure of a molecule and then explain the formation of bonds by the overlap of atomic orbitals.
If our use of simple atomic orbitals does not explain the observed shape of a molecule, we then resort to the concept of hybridized atomic orbitals.
VB theory and
The Lewis structure of

The electron configuration of
Thus, we explain the
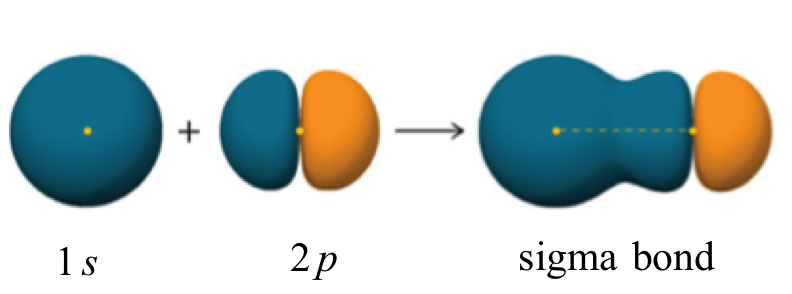
Since there are only two atoms, the molecule is linear, and we don't have to use hybridization to explain its shape.
VB theory and
The Lewis structure of
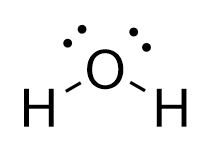
The valence electron configuration of
Thus, we could explain the
The problem is that this predicts the
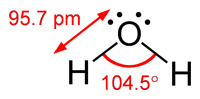
VSEPR theory gives a better prediction of the bond angle.
The four electron pairs repel each other to the four corners of a tetrahedron, for which the theoretical bond angle is 109.5°.
The four hybridized
We explain the 5° difference from theoretical as resulting from repulsion by the lone pair electrons.


