Water can be present in our atmosphere as a solid liquid, or gas. Water freezes at 32 F and vaporizes at 212°F. What is the range of temperatures in which water is not a liquid?
2 Answers
At normal atmospheric pressures water is not a liquid from 0’ Kelvin to 273’K, and from 373’K (100’C, 212’F) and higher.
Explanation:
A phase diagram is a convenient way to see the temperature and pressure dependence of water. It is very important to state the pressure conditions as well, because it will affect the physical state of the water.
And extensive phase diagram and description can be found here:
http://www1.lsbu.ac.uk/water/water_phase_diagram.html
A simpler one for both water and carbon dioxide is here:
http://www.columbia.edu/itc/chemistry/environmental/lectures/week2.pdf
At 1 Atmosphere pressure HOH(s) => T < 0 deg-Celcius; HOH(g) => T > 100 deg-Celcius. If surrounding atmospheric pressure is changed these ranges can change.
Explanation:
The is best illustrated using the 'Phase Diagram for Water'...
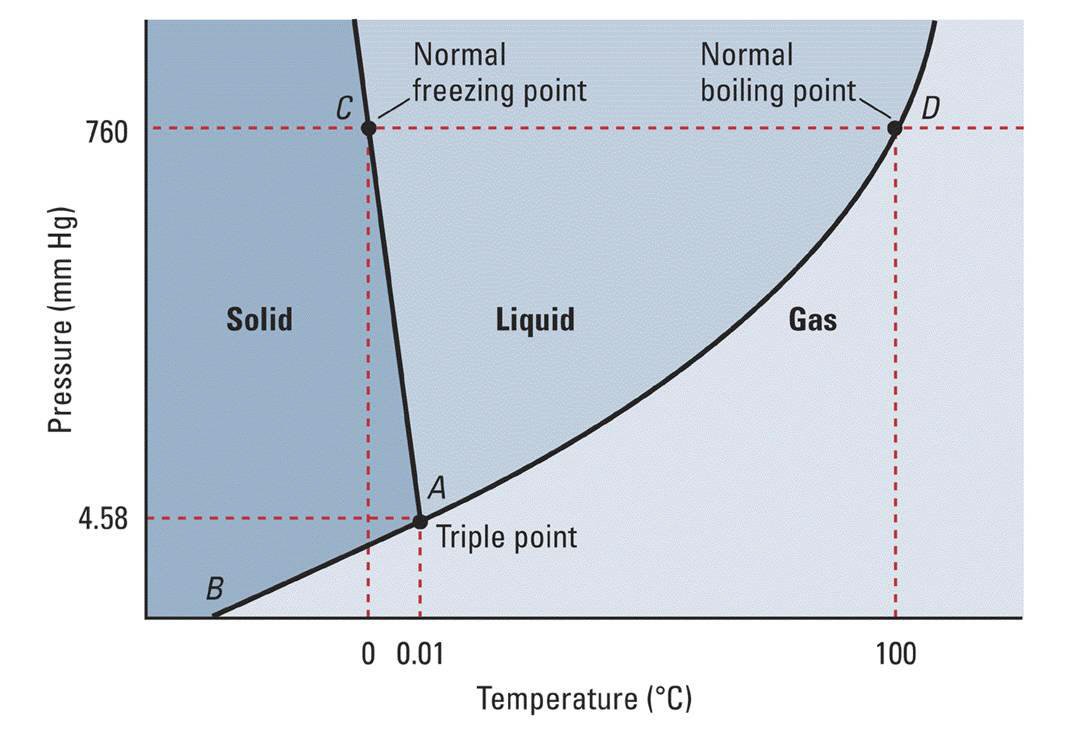
On the 'Pressure' axis note 760 mm. This is 1 Atm. Pressure. The horizontal dashed line passes through the graph at point C and D. These are phase change points at the specified pressure. For point C, this is where water undergoes phase transition from solid to liquid or visa versa. Drop down to the 'Temperature' along the vertical dotted line from C to find the transition temperature. At temperatures below this value (T < 0 deg-Celsius) water will be a solid.
The same procedure for temperature range for water in gas phase can be used to define where water is a gas. Drop down from point D to the temperature axis. At temperatures above 100 degrees Celsius water transitions into the vapor phase as a gas and remains in the gas phase until cooled to a temperature below 100 degrees Celsius.
One should note that if the atmospheric pressure changes, so do the temperature ranges for solid and gas phase transitions.


