Example: Ethane: #CH_3-CH_3#. When ethane reacts with others alkyls(example: Propane), could have a collision between one ethane molecule and one propane molecule, so that one hydrogen breaks from each molecule and the free radicals from each molecule pull the other, creating one molecule. Look:
#CH_3-CH_3 + CH_3-CH_2-CH _3#. One #H# bonded to one carbon in the Ethane colides with one #H# from the middle carbon in the Propane, creating an Ethane and a Propane with free radicals and 2 unstable #H#(because of the free radicals):
#CH_3-CH_2 * + CH_3-CH * -CH_3 + H * + H * # (Those little dots are free radicals) and the carbon chains bond together, as the hydrogen atoms also does it:
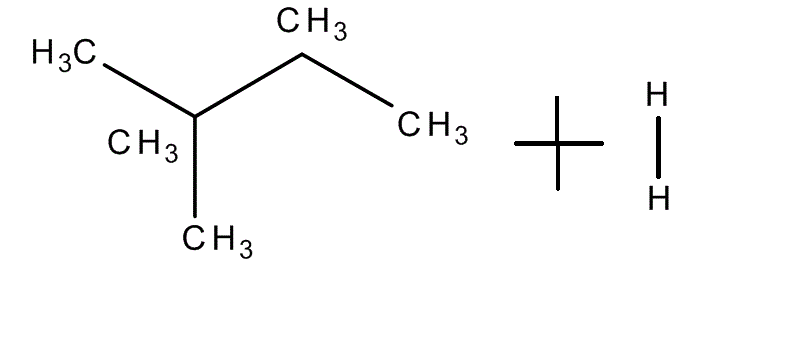
After the bonding of the two carbon chains, now we select a "main chain", with the larger number of carbon atoms(different rules if we have #pi# bonds). So, in this example, we have four atoms in the main chain and one "extra" carbon atom(the radical, the one that goes down in the image). So, the radical is bond to the second atom of the main chain, and has only one atom of carbon(meth+yl -termination for radicals). The main chain has 4 atoms (butane).
So, for the nomenclature, we put it all together: 2-methyl-butane.


