What are cis and trans geometric isomers?
1 Answer
Feb 9, 2016
These isomers occur around double
Explanation:
While everything around a single
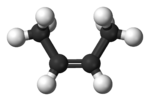
or on the opposite side of each other (trans-) :
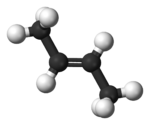
Since there is no rotation possible around the double bond (in the middle) these two can not be converted into one another.
Extra
Instead of using the prefixes cis- and trans- the use of the prefixes Z- and E- are in common use.
The lower picture would be called trans-but-2-ene or E-but-2-ene
(and since the cis/trans can only happen if the double bond is at place 2, the 2 is often omitted, so trans-butene or E-butene)

