What are examples of geometric isomers?
1 Answer
Well, cis-trans 2-butylene for a start....
Explanation:
By specification, geometric isomers are characterized by equivalent connectivity...but different geometry. And
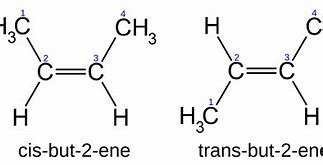
Now while the connectivities of these isomers are manifestly the same, and you can see this in the numbering scheme, their chemistry, and physical properties are distinct. The cis isomer has a normal boiling point of
And disubstituted ring systems, i.e.
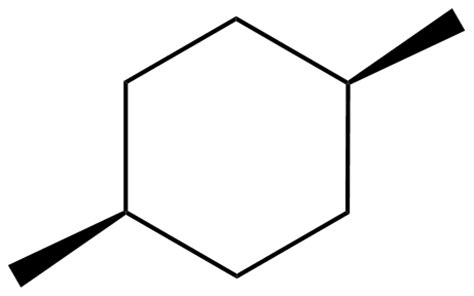
...and this is the

