What are physical properties that can separate a mixture?
1 Answer
Examples might include
- Soluability
- Immiscibility
- Boiling point
Explanation:
Table salt
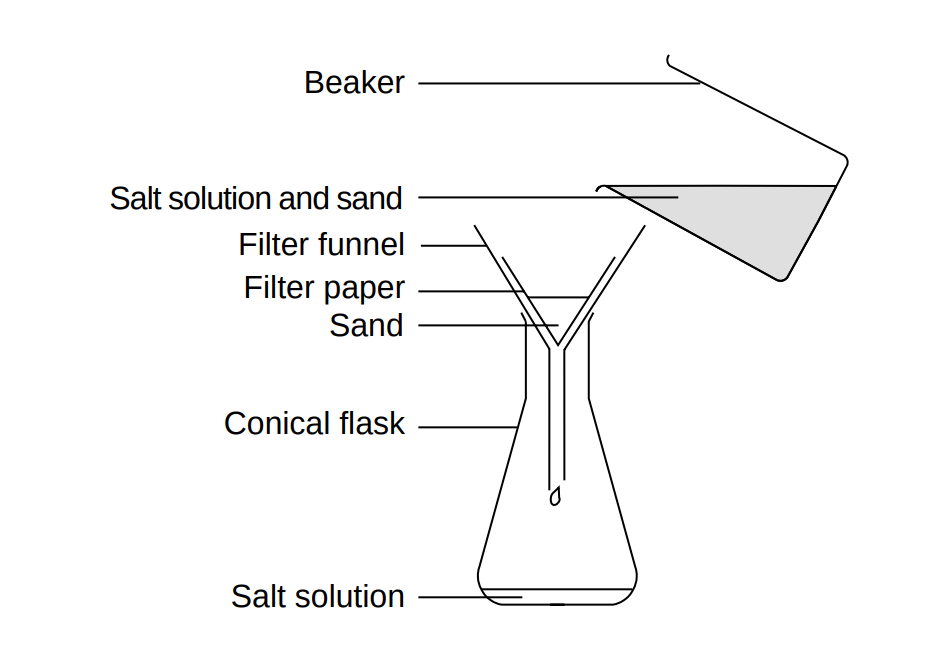 http://www.rsc.org/learn-chemistry/content/filerepository/CMP/00/000/455/CCE-1-SeparatingASandAndSaltMixture.pdf
http://www.rsc.org/learn-chemistry/content/filerepository/CMP/00/000/455/CCE-1-SeparatingASandAndSaltMixture.pdf
For example, to separate a mixture of sand and table salt, start by dissolving the solid mixture in water to form a
Separation funnels (see diagram below) facilitates the separation of two immiscible liquids - liquids that don't dissolve in each other e.g., water and mineral oil.
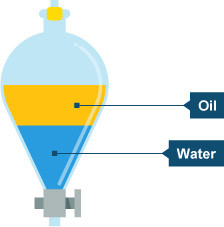 http://www.bbc.co.uk/schools/gcsebitesize/science/add_edexcel/covalent_compounds/seperationrev1.shtml
http://www.bbc.co.uk/schools/gcsebitesize/science/add_edexcel/covalent_compounds/seperationrev1.shtml
What if the two liquids dissolve in each other in significant amounts? Fractional distillation separates substances of different boiling points. For example, it is possible to isolate ethanol
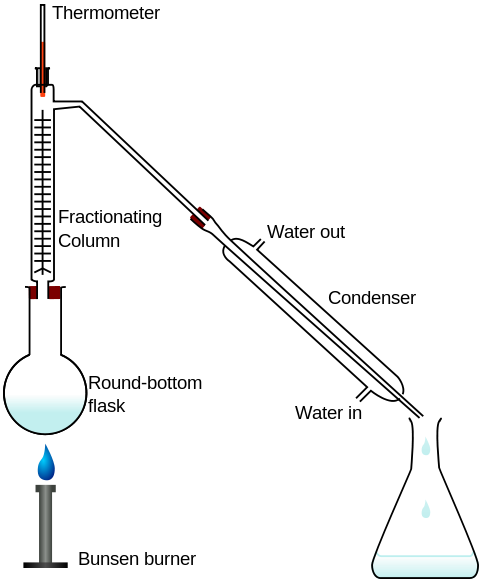 https://commons.wikimedia.org/wiki/File:Fractional_distillation_lab_apparatussvg
https://commons.wikimedia.org/wiki/File:Fractional_distillation_lab_apparatussvg
In the particular setup shown in the diagram, the temperature of the mixture in the round-bottom flask is maintained between

