What are some examples of inorganic chemistry?
1 Answer
Mar 31, 2016
Inorganic Chemistry is actually quite diverse, and generally covers the chemistry of transition metals (orbital diagrams, mechanisms, spectra, etc) and main group elements, but I can show some pretty cool select images from my textbook too.
GENERAL OVERVIEW
Some things you might learn in Inorganic Chemistry:
- Abundance and elemental form of certain elements in nature
- General rules for polarizability (size and electronegativity, generally), reactivity (Hard-Soft Acid-Base Theory), ionic character (ratio of radii, magnitudes of charge, etc), and so on
- Crystal lattice structures and their general makeup (cubic close-packed, hexagonal close-packed, face-centered cubic, etc)
- Thermochemistry (Hess's Law, hydration and lattice enthalpies, etc)
- Electrochemistry (redox reactions, disproportionation and conproportionation reactions, Pourbaix, Frost, and Latimer diagrams, etc)
- Acid naming and predicting acid strength (oxoacids, hydrohalogenic acids, etc)
- Transition metal complexes and their colorful nature
- Crystal Field Theory (
#pi# donors,#sigma# donors,#pi# acceptors, stronger-field/weaker-field ligands, d-orbital splitting diagrams [octahedral, tetrahedral, square planar], etc)
and more!
COOL STUFF IN LATER YEARS
For fun, here's a revolutionary compound called a carbide cluster you might learn about maybe two or three years afterwards.
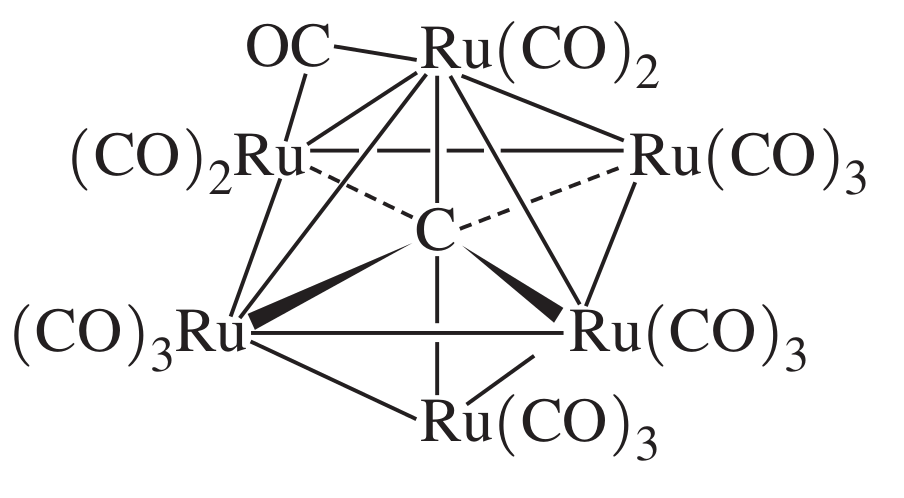
Some neat aspects:
- Notice how carbon is making more than 4 bonds! (Your guess is as good as mine as to how.)
- There's a bridged
#\mathbf("C"="O")# on the upper-left part of the compound connected to two#"Ru"# at once. It is interacting with both via its two antibonding#pi^"*"# MOs.
Here's a cool titanium sandwich!
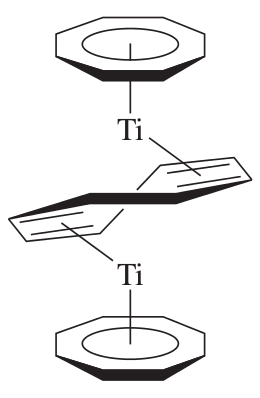
- Titanium is actually interacting with all eight carbons at once on the uppermost and lowermost rings.
- Each titanium is likely also interacting with four carbons at once on the central ring (which I'm assuming is an octatetraene ring).
And finally, for some even weirder stuff, here's a "ring-whizzer" mechanism where a compound gives a single
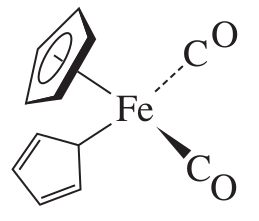
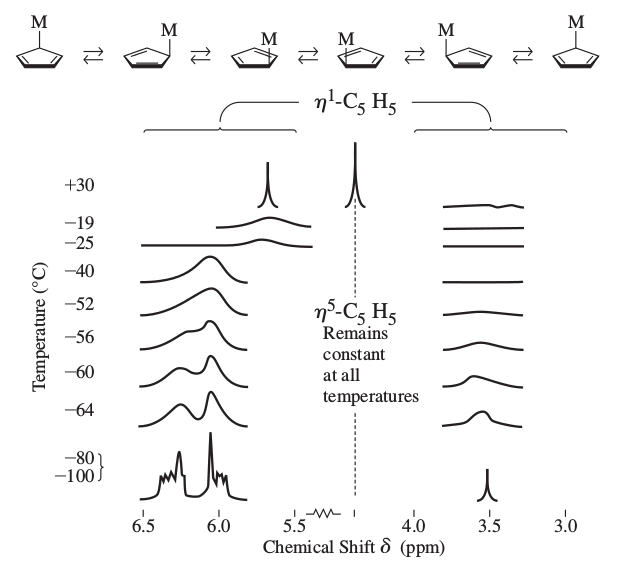
- It generally happens with cyclic ligands that are bound via only one atom to the transition metal (
#eta_1-"C"_5"H"_5# ), where the proton environment keeps changing. At low enough temperatures, this change is detectable, but at high enough temperatures, the signal is an average and we see no peak splitting! - If the ligand is bound by all of its atoms (such as
#eta_5-"C"_5"H"_5# meaning that a cyclopentadiene ring is bound via all 5 carbons), then it's a symmetric bond to the ring, and thus, any "ring-whizzing" retains the same proton environment and no peak splitting is seen.
RADICAL!

