What are the four possible constitutional isomers for the molecular formula C3H5Br?
1 Answer
Jan 9, 2016
Here are the steps I would take to figure them out.
Explanation:
- Decide how many ring or double bonds are in the molecule.
- Draw all the possible acyclic isomers.
- Draw all the possible cyclic isomers.
1. Rings or double bonds
The molecular formula is
Replacing the
The formula of an alkane with 3 carbons is
∴
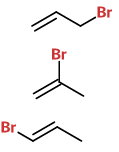
2. Acyclic isomers
(a) Draw all possible alkenes with 3 carbon atoms.
(b) Add a bromine atom in every possible location.
These isomers are 3-bromopropene, 2-bromopropene, and 1-bromopropene.
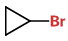
3. Cyclic isomers
(a) Draw all possible cycloalkanes with 3 carbon atoms.
(b) Add a Br atom in every possible location.
This isomer is bromocyclopropane.
And we have our four isomers.