What are the four quantum numbers in chemistry?
1 Answer
Explanation:
Electrons can be described by
The first quantum number is called the principal quantum number, and it's denoted by the letter
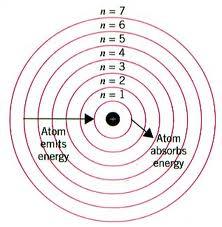
The second quantum number is called the orbital angular momentum number, and it's denoted by the letter
It represents the number of angular nodes (shown in the first image below) in an orbital, which means that it effectively represents the shape of the orbital.
The maximum value of
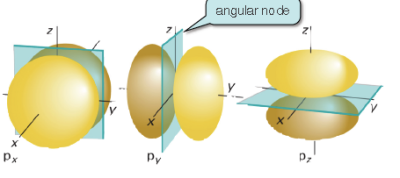
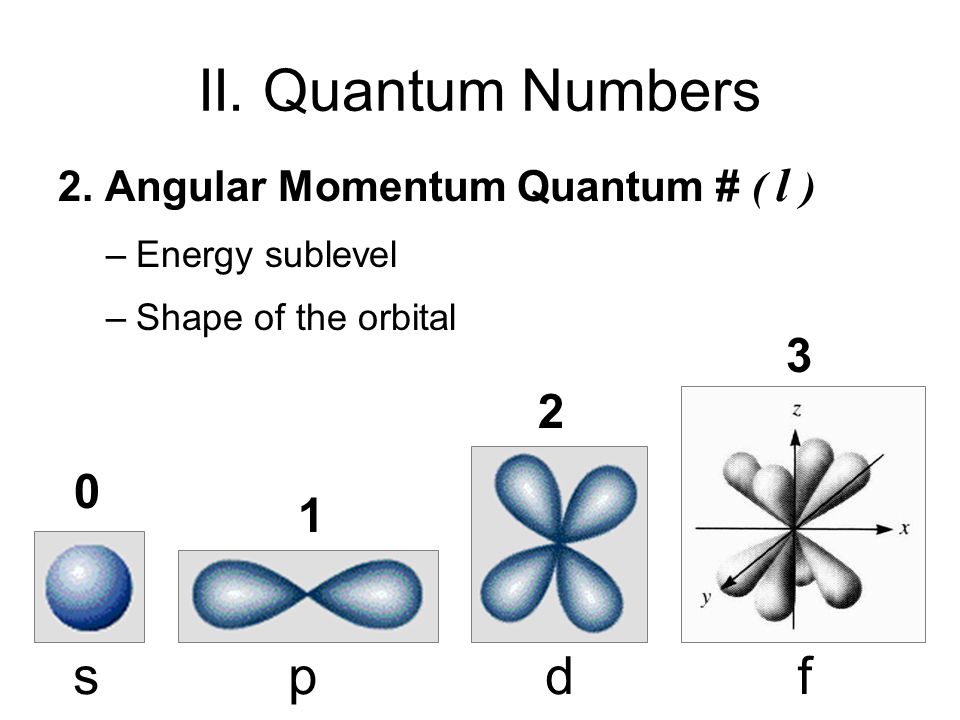
The third quantum number is called the magnetic quantum number, and it's denoted by
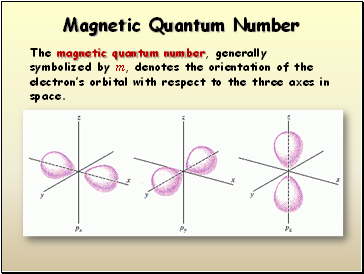
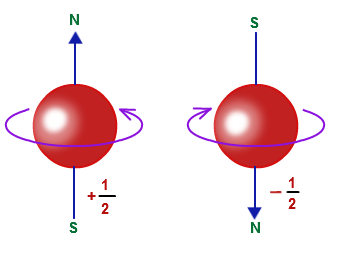
Our fourth quantum number is the electron spin number. It is denoted by
Essentially, every electron has a spin. This a spin is either in the direction of "up" or "down," denoted by the arrows