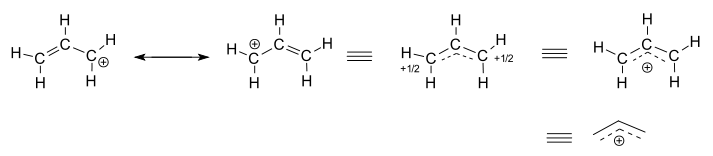
What are the major and minor resonance contributor(s) for the allyl cation?
1 Answer
Feb 7, 2015
The allyl cation has no major and minor resonance structures, it has two resonance structures that contribute equally.
In order to better illustrate why this happens, take a look a the two possible resonance structures for the allyl cation, or
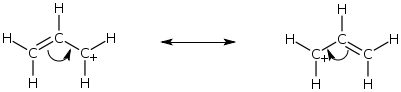
The resonance structure on the left has the positive charge on a carbon atom that does not has a full octet, two things that are usually characteristic of minor resonance structures.
However, the structure on the right is idential to the one on the left from this standpoint. One of the three carbon atoms has an incomplete octet and a positive charge.
This means that these resonance structures will contribute equally to the hybrid structure, as you can see here